R&D Achievements Ahead of 'ARBM-101' Clinical Trials
페이지 정보

본문
ODD Designation from FDA Accelerates Development…Gaining Attention as a 'Complex Generic' Cash Cow
Arbormed, a biotech venture specializing in developing treatments for rare and intractable diseases, has received Orphan Drug Designation (ODD) from the U.S. regulatory authorities ahead of its lead pipeline's entry into clinical trials.
The company’s core competitiveness lies in its ability to achieve both efficacy and safety based on a differentiated mechanism of action in the field of rare diseases, where no fundamental treatments currently exist.
Arbormed has been steadily accumulating research and development (R&D) achievements, supported by a strong cash flow from its complex generic drug business.
The recent ODD designation is expected to accelerate the commercialization of its pipeline, which could, in turn, provide a boost to ongoing discussions about potential technology exports.
Wilson's Disease, Without a Fundamental Cure, Achieves Both Efficacy and Safety Through a New Mechanism
According to the biotech industry, Arbormed’s candidate for the treatment of Wilson’s disease, ARBM-101, recently obtained ODD from the U.S. Food and Drug Administration (FDA).
The FDA’s ODD program is designed to facilitate the development and approval of drugs for rare diseases. Drugs designated as ODD receive benefits such as tax credits for clinical development costs, reduced fees for regulatory review,
and seven years of market exclusivity after approval.
Wilson’s disease, the target indication for ARBM-101, is a rare genetic disorder characterized by an inability to excrete accumulated copper from the body. The excess copper primarily accumulates in the liver, leading to various liver-related complications. Although Wilson’s disease has a relatively higher incidence rate among rare diseases, there is currently no fundamental cure.
The only approved treatment for Wilson’s disease is penicillamine, which was approved by the FDA in the 1970s. However, this drug only prevents further copper accumulation in the liver by promoting diuresis and carries significant risks, including kidney failure. Due to these adverse effects, approximately 30% of patients discontinue drug treatment.
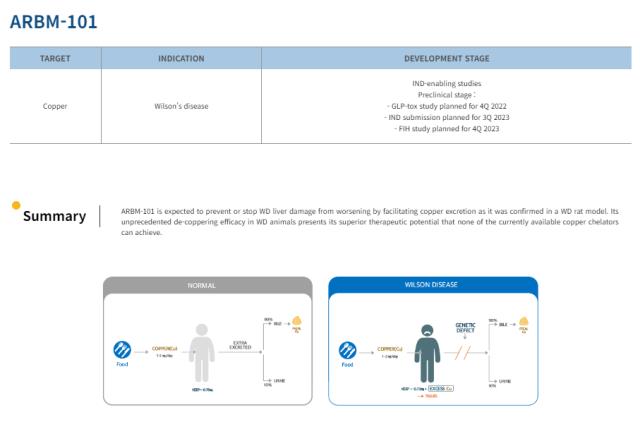
Overview of Arbormed’s Wilson’s Disease Candidate, ARBM-101
ARBM-101 is designed to promote the rapid excretion of copper from liver tissue, targeting the disease's root cause. The drug works by harnessing microorganisms that selectively bind to copper, facilitating its excretion through feces. In preclinical studies conducted on animal models of acute and chronic liver disease caused by Wilson’s disease, ARBM-101 demonstrated significantly faster copper excretion compared to control groups. The pharmacological efficacy of minimizing side effects through fecal excretion was also confirmed.
Given its novel mechanism of action, ARBM-101 is being developed as a first-in-class drug, with plans to enter Phase 1 clinical trials in multiple countries next year. With the recent ODD designation, the timeline for commercialization has been significantly shortened.
R&D-Backed Cash Cow 'Complex Generic'…Pursuing Licensing Out Before Phase 1
Arbormed has been consistently achieving R&D milestones. The company presented the results of its ARBM-101 research at the European Wilson’s Disease Conference (Aarhus) and the American Association for the Study of Liver Diseases (AASLD). Additionally, ARBM-101 was selected for presentation at the European Association for the Study of the Liver (EASL) last year. The drug was also chosen as a research support project by the Korea Drug Development Fund (KDDF) last year.
These achievements have been made possible by a solid cash flow foundation. Complex generics refer to pharmaceutical products that are difficult to manufacture and develop, limiting the number of generic products available. With limited competition, these products guarantee profitability. Abomed has capitalized on this by obtaining U.S. regulatory approval for complex generics and building a business model that imports and distributes finished pharmaceuticals.
The first product expected to enter the market is a general anesthesia adjuvant from Korea's Penmix, currently under review for Common Technical Document (CTD) compliance, with plans to file for marketing approval in the first quarter of next year. Arbormed is also planning to file for ANDA approval for this complex generic. An injectable treatment for severe osteoporosis from India’s Etico Life Sciences is also expected to begin the approval process in the second quarter of next year.
With continued progress in R&D, ongoing discussions regarding technology exports are expected to gain momentum. The company is reportedly in active communication with global companies and is exploring the possibility of licensing out the technology before entering Phase 1 clinical trials. Arbormed is considering various strategies, including seeking funding for clinical trials to develop the drug in-house or fully outsourcing the clinical trials to a licensing partner.
An Arbormed representative stated, "The ODD designation for ARBM-101 marks the first step toward FDA approval," adding, "We are also preparing to apply for Orphan Drug Designation from the European Medicines Agency (EMA) to further enhance the efficiency of the clinical trial process."
관련링크
댓글목록
등록된 댓글이 없습니다.

